In the current COVID-19 pandemic, a desperate hunt for a vaccine was prevalent to mitigate the health crisis. To address this global demand for SARS-CoV-2 vaccines, there are several vaccine candidates under the draft landscape of the World Health Organization (WHO) [1]. Scientists at the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV), in collaboration with Bharat Biotech, presented the world with the first Indigenous vaccine, made in India; COVAXIN [2]. This vaccine made news when it (along with COVISHIELD, developed by AstraZeneca – Oxford University) was granted restricted emergency use. The Drug Controller General of India approved these vaccines for healthcare and frontline workers on January 3rd, 2021 [3], albeit without phase III efficacy data.
Now, the efficacy of the vaccine is creating news. The interim analysis of the phase III trial by Bharat Biotech, manufacturer of COVAXIN, suggests the efficacy of the vaccine is 80.6% [4] (yet to pass through the peer review). This means that COVAXIN, after two doses, is as good as 81% in preventing COVID-19 in individuals without a previous history of infection.
A decisive turning point to strengthen a spirited fight!
— Narendra Modi (@narendramodi) January 3, 2021
DCGI granting approval to vaccines of @SerumInstIndia and @BharatBiotech accelerates the road to a healthier and COVID-free nation.
Congratulations India.
Congratulations to our hardworking scientists and innovators.
A decisive turning point to strengthen a spirited fight!
— Narendra Modi (@narendramodi) January 3, 2021
DCGI granting approval to vaccines of @SerumInstIndia and @BharatBiotech accelerates the road to a healthier and COVID-free nation.
Congratulations India.
Congratulations to our hardworking scientists and innovators.
On Monday 08th, March 2021, the Phase II clinical trial data from COVAXIN (BBV152) was available online [5]. Published in the prestigious peer-reviewed journal, The Lancet Infectious Diseases, this study evaluated the immunogenicity and safety of BBV152 at two different vaccine formulations. (This was based on the Phase I clinical data [6])
The Vaccine
The SARS-CoV-2 virus strain NIV-2020-770 (dominant strain in the pandemic with A614G mutation) was isolated from a COVID-19 patient and sequenced at ICMR-NIV [7]. This was supplied to Bharat Biotech, which, using their biosafety level 3 manufacturing facilities and Vero cell manufacturing platform, rapidly developed BBV152, a whole virion SARS-CoV-2 vaccine [6].
Unsolicited adverse events were recorded post-vaccination for safety assessments. For Immunogenicity assessments, participants were kept under medical observation for 2 hours on-site [5, 6].
Methods:
This was a randomized, double-blind, multicenter, phase II clinical trial. It evaluated the safety and immunogenicity of COVAXIN in healthy adults and adolescents aged range of 12 to 65. The study excluded the participants positive for SARS-CoV-2 in RT-PCR or serological tests.
- Randomized clinical trials: subjects are randomly assigned to one of the treatment regimens and then treated in an identical manner
- Double-blind clinical trials: Researchers or the patients are unaware of the treatment regimen being administered
- Multicenter clinical trials: Conducted at more than one registry center; the case subjects were recruited at nine hospitals in India
This multicentric phase II trial involved 380 participants (out of 921 that were screened) who were equally and randomly segregated into two groups (180 each). These groups had two dosing regimens – 3 and 6 micrograms, both with the same adjuvant (Algel-IMDG [8, 9], which is designed to carry the vaccine directly to draining lymph nodes, surpassing the systemic circulation. Adjuvants are incorporated with the vaccines to elicit a boosted immune response [10].
To ensure commonality with other licensed COVID-19 vaccines, a 28-day interval between the two doses was scheduled in contrast to 14 14-day apart dosing schedule in the Phase I trial. This trial excluded the placebo groups with an increased sample size [5].
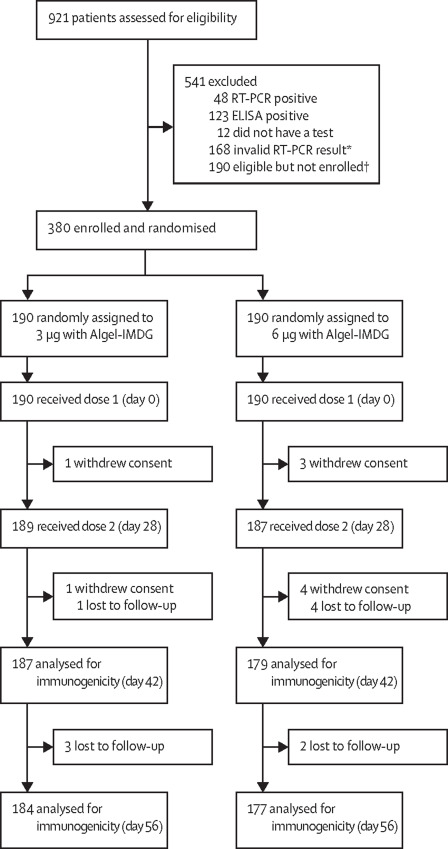
Findings:
Using ELISA, anti-IgG responses against the spike (S1) glycoprotein, receptor-binding domain (RBD), and nucleocapsid protein were assessed.
The vaccine was well tolerated in both treatment regimens with no life-threatening events. Men and women receiving similar vaccines had no immunological differences across age groups. Interestingly, fewer participants had an adverse event in the phase II trial than in phase I. BBV152 also exhibited an enhanced humoral and cell-mediated immune response in comparison to the Phase I trial. Neutralizing antibody titres after the phase II trial were similar to those observed in a panel of convalescent serum samples.
“Long-term follow-up of phase I trial participants showed that neutralizing antibody titres persisted, and T-cell memory responses were more pronounced in the 6 µg with Algel-IMDG group compared with pre-vaccination samples.” Authors presented.
96.6% of the participants receiving the 6-µg vaccine elicited an immune response compared to 88% of participants with the 3-µg vaccine regimen. This formed the basis of the selection of the 6-µg vaccine in the phase 3 efficacy trial.
Good News: Phase 2 Results of COVAXIN (Bharat Biotech) Published in Lancet
— Faheem Younus, MD (@FaheemYounus) March 9, 2021
380 volunteers studied
Double-blind, RCT trial
2 shots – 28 days apart
No serious (level 4/5) side effects
Can't determine efficacy by phase 2 trials but it's safe/ immunogenic https://t.co/Ef2pdrzZ8c pic.twitter.com/AtXakkJyz6
A massive phase III efficacy trial involving 25800 volunteers is carried out with 6 µg of BBV152 Algel-IMDG formulation. Dr Krishna Ella, Chairman and Managing Director, Bharat Biotech, while announcing the phase III trial reports, assured that COVAXIN provides significant immunogenicity against the rapidly emerging SARS-CoV-2 variants [4]. It is also claimed that COVAXIN can neutralize the UK variant strain of SARS-CoV-2 [11].

To conclude, the candidate vaccine (COVAXIN) is found to be safe, immunogenic, and can be stored at 2-8°C, matching the cold chain storage compatibility of most countries. The lack of ethnic and racial diversity in the phase II trial data, however, warrants evaluation of BBV152 in other populations for wider applicability. Further studies are underway to assess efficacy and immune responses in children, older individuals, and people with comorbidities.
Finally, the vaccine war is a real thing!!
Vaccine wars pic.twitter.com/TpEl3yILv8
— TwistedDoodles (@twisteddoodles) November 18, 2020
Om Sarve Bhavantu Sukhinah!!
© Tryambak Srivastava
March 11, 2021
New Delhi, Delhi, India
Footnotes:
- WHO Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- COVAXIN® – India’s First Indigenous COVID-19 Vaccine https://www.bharatbiotech.com/covaxin.html
- Press Statement by the Drugs Controller General of India (DCGI) on Restricted Emergency approval of COVID-19 virus vaccine https://pib.gov.in/PressReleseDetail.aspx?PRID=1685761
- Bharat Biotech Announces Phase 3 Results of COVAXIN®: India’s First COVID-19 Vaccine Demonstrates Interim Clinical Efficacy of 81% https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-results.pdf
- Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00070-0/fulltext
- Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30942-7/fulltext
- First isolation of SARS-CoV-2 from clinical samples in India https://www.ijmr.org.in/article.asp?issn=0971-5916;year=2020;volume=151;issue=2;spage=244;epage=250;aulast=Sarkale
- Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease https://www.nature.com/articles/nm.1894
- Potent Adjuvanticity of a Pure TLR7-Agonistic Imidazoquinoline Dendrimer https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0043612
- Alum adjuvant discovery and potency https://www.nature.com/articles/d42859-020-00011-w
- Neutralization of UK-variant VUI-202012/01 with COVAXIN vaccinated human serum https://www.biorxiv.org/content/10.1101/2021.01.26.426986v2