ABSTRACT:
Gomes et al. report that MMA levels are significantly higher in the blood of healthy people over the age of 60 than in those under 30. The elevated level of MMA had not caused ill health in the individuals studied. However, the authors found that treating human cancer cells with serum from the blood of the older group, or with high concentrations of MMA, led them to adopt characteristics of metastatic cancer cells — those that can spread from a primary tumour to seed cancers elsewhere in the body. These characteristics include a loss of cell–cell attachment and an increase in mobility. When injected into mice, the cells formed metastatic tumours in the lungs.

INTRODUCTION:
Epithelial-mesenchymal transition (EMT)
EMT is a biological process that allows a polarized epithelial cell, which normally interacts with basement membrane via its basal surface, to undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype [4]. The epithelial and mesenchymal cell markers are listed. Colocalization of these two sets of distinct markers defines an intermediate phenotype of EMT, indicating cells that have passed only partly through an EMT. The completion of an EMT is signaled by the degradation of underlying basement membrane and the formation of a mesenchymal cell that can migrate away from the epithelial layer in which it originated.

This includes enhanced migratory capacity, invasiveness, elevated resistance to apoptosis, and greatly increased production of ECM components [4]. EMT is a developmental concept to generate more cells and expand tissue size often highjacked by cancer cells to acquire pro-metastatic properties [5].
Methylmalonic acid (MMA)
Since authors show that methylmalonic acid (MMA), a by-product of propionate metabolism, is upregulated in the serum of older people and functions as a mediator of tumour progression, let us walk through the basics of MMA. It is a dicarboxylic acid that is primarily produced as a by-product of propionate metabolism yielding from the catabolism of branched-chain amino acids and odd chain fatty acids. Usually, MMA becomes linked to the coenzyme A to form methylmalonyl-CoA, which is converted to succinyl-CoA in a reaction that involves vitamin B12 as a cofactor. Succinyl-CoA subsequently enters the TCA cycle — a series of chemical reactions that are a key part of energy production in the cell. Toxic accumulation of MMA in the blood leads to metabolic disorder methylmalonic acidaemia [6].

Considering the growing body of evidence that cancer cell-extrinsic factors are key in modulating tumour progression, authors hypothesized that Ageing might produce a systemic environment that supports tumour progression and aggressiveness
They laid this AIM to achieve for their study…
To identify the cancer cell-extrinsic factors responsible for modulating tumour progression
To fulfil their AIM, authors laid the following objectives…
- To assess the phenotypic changes in cancer cells grown in human sera
- To identify age-induced circulatory factors that promote cancer aggression
- To understand the pro-aggressive properties of circulatory factors
- To identify the intracellular delivery and regulatory role of circulatory factors
- To understand pro-aggressive transcriptional reprogramming induced by the circulatory factors
METHODOLOGY:
Human serum from 30 ‘young’ (aged 30 and below) and 30 ‘old’ (aged 60 and above) male adults with no diagnosed disease at the time of collection were obtained. The race of donors is indicated as A, African American; C, Caucasian; H, Hispanic.
The cell lines used were:
A549 are are adenocarcinomic human alveolar basal epithelial cells from 58 yo caucasian male. It is a model for non-small cell lung cancer.
HCC1806 human breast cancer (triple negative breast cancer; TNBC) from 60 yo black female. It is poorly differentiated negative for ER/PR/Her2 and p53.

RESULTS:
Whereas the majority (25 out of 30) of cells treated with young donor serum maintained their epithelial morphology, cells treated with 25 out of the 30 old donor sera became mesenchymal, losing their polarity and displaying a spindle-shaped morphology. These phenotypes were independent of donor ethnicity, and resembled the epithelial-to-mesenchymal transition (EMT), as exhibited by metastatic cancer cells.



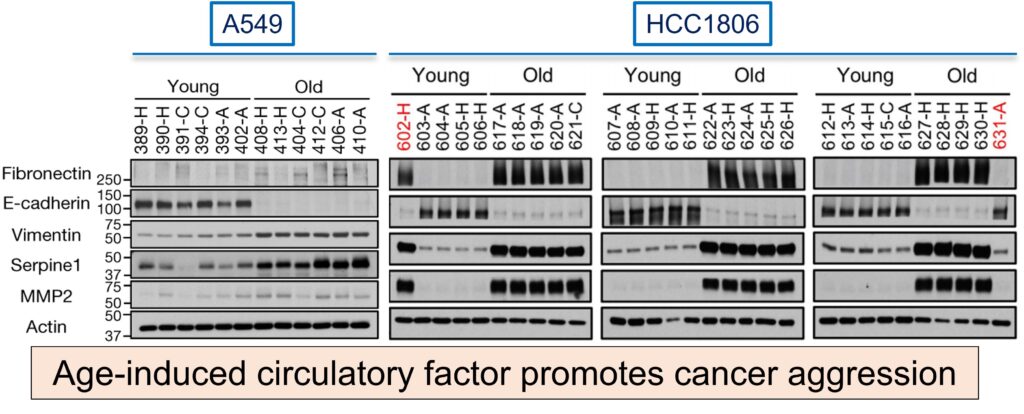
To correlate the Epithelial to Mesenchymal phenotype, authors performed western blotting on A549 and HCC1806 cell lines cultured for 4 days in HS from young or old donors with EMT markers. Cells cultured with aged-donor serum displayed a pronounced loss of the epithelial marker E-cadherin and gain of the mesenchymal markers’ fibronectin and vimentin, in addition to increased expression of serpine1 and MMP2—proteins associated with aggressive phenotypes.
Cells were seeded in 96-well plates in technical triplicates. These were treated next day with either vehicle control (DMSO (0.1%)), carboplatin (0–200 μM), or paclitaxel (0–7.5 nM). The aged sera promoted resistance to two distinct and widely used chemotherapeutic drugs, carboplatin and paclitaxel.

Will the cells treated with the old sera also show elevated metastatic potential?
For bioluminescent tracking, MDA-MB-231 cell lines were retrovirally infected with a reporter construct encoding firefly luciferase producing bioluminesence. luc-positive cells were enriched by fluorescence-activated cell sorting. MDA-MB-231 cells were treated with HS and then injected into the tail veins of nude mice for evaluation of lung colonization. Lung colonization assay is performed because lungs and bones are the preferential choice of BrCa cells to metastasize first followed by brain. Female athymic mice were injected with 100,000 cells in 100 μl PBS into the tail vein. Metastases were monitored using in vivo Imaging System. Then luminescence was measured and quantified.

Before performing LCA on MB231 cells, authors replicated the EMT WB studies first on MDA-MB-231-luciferase cells cultured for 4 days in HS from young or old donors. This showed similar pattern of EMT markers’ expression as by NSCLC and TNBC. In contrast to the young sera, the aged sera has statistically significant luminous intensity and robustly potentiated the ability of the cells to colonize the lungs and form metastatic lesions. So, it was validated that there is a link between systemic ageing and age-induced circulatory factors that promote the acquisition of aggressive properties of cancers.

Next, they aimed to identify age-induced circulatory factors that promote cancer aggression. There must be something in the aged sera that elevated cancer aggression. In the reported literature, it is stated that metabolic interventions such as diet, exercise and caloric restriction influence the outcomes of cancer. So, authors wanted to assess the metabolic compositions of the donor sera for which they employed targeted metabolomics.
179 circulatory metabolites were detected out of which only 10 were altered at a statistically significant level. Notably, only three metabolites were consistently increased in the sera of aged donors: phosphoenolpyruvate, quinolinate, and methylmalonic acid (MMA).
To test whether any of these three metabolites was responsible for inducing the pro-aggressive effects, they treated A549 cells with each metabolite. It was observed that only MMA replicated the similar results. Notably, the pro-aggressive effects of MMA were specific, as different acids with similar structures and pKa values did not induce the same response to EMT markers as previously exhibited. Hence, MMA is the age-induced circulatory factor found in the aged sera.



Now that the age-induced circulatory factor is identified, next authors wanted to understand the pro-aggressive properties of MMA.
To better understand the pro-aggressive properties of MMA, they treated HCC1806, A549 and MCF-10A cells (a is a non-tumorigenic epithelial cell line derived from breast fibrocystic disease and a common model for EMT studies) with gradual conc. of MMA. In the MMA stimulation studies in these three cell lines, it was observed that concentrations of 1 mM and above are sufficient to induce EMT-like phenotype and the expression of pro-aggressive proteins.
Next in line authors performed chemotherapeutic drug assays on A549 and HCC1806 cells. They found that MMA also induced resistance to carboplatin and paclitaxel as previously with aged sera.


Transwell migration assay of A549 cells treated with 5 mM MMA for 10 days. Treatment of MDA-MB-231 cells in vitro with MMA increased markers of aggressiveness and was sufficient to robustly increase the ability of the cells to colonize the lungs of athymic mice in a concentration-dependent manner. It was concluded that MMA promotes pro-aggressive traits and contributes to the cellular plasticity required for tumour progression.

Discrepancy in MMA induced effects
Although MMA concentrations above 1 mM were required to potently induce aggressive traits in cancer cells in vitro, MMA concentrations measured in serum from old donors were much lower than this. Further analysis demonstrated that the intracellular concentrations of MMA achieved within 4 h of treatment with old serum or 5 mM MMA were substantially different. In fact, 5 mM MMA took 48 h to produce intracellular concentrations similar to the ones observed after 4 h treatment with old serum. Suggesting that the discrepancy in concentrations is because added MMA has a lower cell permeability than endogenous MMA in donor serum. A more cell-permeable version of MMA (dimethyl MMA) could induce pro-metastatic effects at concentrations as low as 10–50 μM.

Is there another component of the serum that could facilitate the entry of MMA into cancer cells? depleted the old serum of lipids or of molecules larger than 3 kDa—two manipulations that should not affect the levels of polar metabolites such as MMA. Strikingly, both manipulations also caused a pronounced decrease in serum MMA levels. MMA responsible for this phenotype is complexed with molecules larger than 3 kDa in the serum that facilitate its entry into cancer cells.

Identifying the lipidic structures complexed with MMA
Next, they targeted to identify the lipidic structures complexed with MMA. To test that hypothesis, they first complexed MMA with synthetic lipidic structures (lipofectamine, a common transfection reagent) or with lipidic structures purified from FBS. With both approaches, the concentration of MMA necessary to induce pro-aggressive properties reached the levels similar to that of the old donor serum. In fact, MMA complexed with lipidic structures from FBS produced a similar intracellular concentration of MMA within the same time frame as treatment with old donor serum.
Treatment of cancer cells with lipidic structures isolated from old serum, or from young serum, or isolated from young serum and loaded with MMA at concentrations similar to the ones found in the old serum, was sufficient to drive pro-aggressive properties. Also, depletion of lipidic structures from old serum resulted in a reduction in total serum MMA levels and was sufficient to abrogate the pro-aggressive phenotype. MMA, complexed with lipidic structures, is a circulatory factor that contributes to the pro-aggressive effects of ageing in cancer cells and is sufficient to drive tumour progression and aggressiveness.


The big question, that lies is How MMA promotes the observed cellular plasticity?
MMA induced marked transcriptional reprogramming. The gene set enrichment analysis yielded that MMA regulates genetic programs associated with cell fate decisions, such as wound healing and pattern specification as well as angiogenesis. GSEA also showed that MMA positively regulates transcription, suggesting that the observed pro-aggressive transcriptional reprogramming may be mediated through this role.
Many of the upregulated genes encode secreted proteins known to remodel the tumour microenvironment, including factors that promote reorganization of the extracellular matrix, immunosuppressive cytokines, and ligands that promote cell-to-cell communication.


To find the transcriptional regulators involved, they again performed global transcriptomic analysis at an earlier time point during MMA treatment (day 3).
9 of these TFs were significantly changed when validated with quantitative PCR. One of the genes most highly upregulated in response to MMA was SOX4, which encodes a transcription factor involved in the regulation of embryonic development and cancer progression and is known to be a master regulator of EMT.
So, to check its expression at proteomic level, they performed WB. The levels of SOX4 were considerably increased in a variety of cell models treated with different concentrations of MMA, as well as in cells cultured with aged serum. A comparison between genes induced by SOX4 and those induced by MMA treatment revealed a statistically significant overlap of 199 genes. Functional annotation reveals that the overlapping genes—are associated with pro-aggressive genetic programs.
This supports the idea that SOX4 mediates the pro-aggressive phenotype observed.

To better understand the relationship between MMA and SOX4, they used short hairpin RNA (shRNA) to suppress SOX4 expression. shNT: non-specific shRNA. Suppression of SOX4 blocked the ability of MMA or old serum to induce EMT and aggressive markers. Finally, SOX4 depletion fully abrogated the ability of MMA to promote migratory and invasive properties or resistance to chemotherapeutic drugs.
The authors demonstrated that repressing SOX4 expression blocked the ability of MDA-MB-231-luciferase cells to form colonies in the lungs of athymic mice upon treatment with MMA or with old serum.


How MMA alters SOX4 levels?
TCA-related metabolites regulate transcription by modulating levels of histone methylation. RNA-seq analysis revealed an increase in the TGFβ signalling pathway components, including the upregulation of TGFβ-2 ligand. however, treatment with MMA did not change total levels of major histone modifications.
The authors hypothesized that MMA activates transcription of the gene TGF-β2; this gene is part of a TGF-β signalling pathway that, in turn, promotes SOX4 expression. They confirmed the increase in the mRNA of TGFB2 by qPCR, which correlated with an increase over time in the abundance of TGFβ-2 in the medium of cancer cells treated with MMA
Time-course analysis showed that MMA robustly induced TGFβ signalling within 24 h, during which an increase in SOX4 was observed, before any of the pro-aggressive markers were detected, suggesting a link between activation of TGFβ signalling, SOX4 induction and the acquisition of pro-aggressive properties driven by MMA. To test whether activation of TGFβ signalling is responsible for upregulation of SOX4, They treated cancer cells with MMA and a TGFβ receptor. Inhibition of the TGFβ receptor was sufficient to block the ability of MMA to induce SOX4 expression and pro-aggressive properties.
MMA relies on the activation of TGFβ signalling to induce SOX4 which consequently does transcriptional reprogramming to confer cellular plasticity to sustain tumour progression.


Gomes et al. report that MMA levels are significantly higher in the blood of healthy people aged above 60 than in those under 30. The elevated level of MMA had not caused illness in the individuals studied. However, the authors found that treating human cancer cells with serum from the blood of the older individuals, or with high concentrations of MMA, led them to adopt characteristics of metastatic cancer cells — those that can spread from a primary tumour to seed cancers elsewhere in the body. These characteristics include a loss of cell–cell attachment and an increase in mobility. When injected into mice, the cells formed metastatic tumours in the lungs.
Age-induced accumulation of circulatory MMA induces SOX4 expression through the TGFβ pathway and elicits a transcriptional reprogramming that supports aggressiveness, promoting tumour progression and metastasis formation.

To conclude,
Lipids have dual roles in MMA-driven metastases:
- Precursor of MMA in the form of odd chained fatty acids
- Carrier of MMA as large lipids across the plasma membrane
The people in this study who had high MMA levels were cancer-free. This suggests that the effects of MMA are specific to cancer spread in the body, not to initiate cancer formation.
REFERENCES:
Article based on
Age-induced accumulation of methylmalonic acid promotes tumour progression

Leave a Reply